NMIN
EVENTS
NMIN Lectures
Ongoing
Up-coming
Past
23 May 2024 | NMIN Lecture
30 April 2024 | NMIN Lecture
16 April 2024 | NMIN Lecture
26 March 2024 | NMIN Lecture
3 November 2023 | NMIN Lecture
4 October 2023 | Pieter Cullis Invitational
28 September 2023 | NMIN Lecture
18 July 2023 | NMIN Lecture
24 March 2023 | Pieter Cullis Invitational
16 March 2023 | NMIN Lecture
29 November 2022 | NMIN Lecture
20 September 2022 | Pieter Cullis Invitational
31 May 2022 | Pieter Cullis Invitational - Scientific Integrity Series
20 May 2022 | NMIN Lecture
20 April 2022 | Pieter Cullis Invitational
23 March 2022 | NMIN Lecture
11 February 2022 | NMIN Lecture
28 September 2021 | Pieter Cullis Invitational
21 September 2021 | NMIN Lecture
29 June 2021 | NMIN Lecture
23 March 2021 | Pieter Cullis Invitational
23 February 2021 | NMIN Lecture
No more lectures are being planned…
Questions: contact Divya Rao, Manager, HQP Programs & Network Events, at: divyarao@nanomedicines.ca
NMIN
PAST LECTURES
23 May 2024 | NMIN Lecture
30 April 2024 | NMIN Lecture
16 April 2024 | NMIN Lecture
26 March 2024 | NMIN Lecture
3 November 2023 | NMIN Lecture
4 October 2023 | Pieter Cullis Invitational
28 September 2023 | NMIN Lecture
18 July 2023 | NMIN Lecture
24 March 2023 | Pieter Cullis Invitational
16 March 2023 | NMIN Lecture
29 November 2022 | NMIN Lecture
20 September 2022 | Pieter Cullis Invitational
31 May 2022 | Pieter Cullis Invitational - Scientific Integrity Series
20 May 2022 | NMIN Lecture
20 April 2022 | Pieter Cullis Invitational
23 March 2022 | NMIN Lecture
11 February 2022 | NMIN Lecture
28 September 2021 | Pieter Cullis Invitational
21 September 2021 | NMIN Lecture
23 March 2021 | Pieter Cullis Invitational
23 February 2021 | NMIN Lecture
NMIN
NMIN Lectures
Plasmonic nanoparticles for use in nanomedicine
Dr. Michel Meunier
Professor of Engineering Physics and Biomedical Engineering, Polytechnique Montréal
23 May 2024
10 – 11 am PT | 1 – 2 pm ET
Plasmonic nanoparticles such as gold, silver, or their alloys are interesting nanomaterials for application in nanomedicine therapeutics and diagnostics. In this talk, I will briefly introducing plasmonic nanoparticle characteristics, then discuss recent developments in this field at Polytechnique.
Among the developments I will describe is a new method for delivering exogenous biomolecules into targeted cells using an ultrafast laser and plasmonic nanoparticles. We have used the technique of plasmon-mediated laser nanosurgery to effectively perform gene transfection in various living cells and delivery of biomolecules in vivo in animal models for ophthalmic applications. I will share some of our recent results on laser trigger release of antitumor drugs from lipid nanoparticles containing gold nanoparticles.
Moreover, we have synthesized alloy nanoparticles using an improved seeded-growth approach. We use these spectrally distinctive plasmonic nanoparticles as biomarkers to perform quantitative multiplexed 3D imaging of cells and tissues. This technology has been commercialized by our spin-off company, Vega BioImaging, to assist pathologists with their diagnostics.
Our plasmonic-based techniques show promise as innovative tools for basic research in biology and medicine, and could be adapted for use as therapeutic, diagnostic, and theranostic clinical tools.
Dr. Michel Meunier obtained a PhD from MIT in 1984. He began his career at Polytechnique Montreal in 1985 and was promoted to full professor in 1993. Holder of a Canada Research Chair Tier 1, Michel Meunier is a laureate, in 2006, of a Synergy Award for Innovation and, in 2016, of the Guy Rocher Award for excellence in teaching at the university level. He is a Fellow of the Canadian Academy of Engineering, the Optica Society, and the International Society for Optics and Photonics. His research activities focus on the development of new optical nanomaterials, nano-optical devices, and laser technologies for nanomedicine applications. He has published more than 410 articles and supervised over 150 graduate students and postdoctoral fellows. He is the co-founder of Vega BioImaging.

The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lectures
Porphysome Nanotechnology: Beyond Lab, Beyond Light and Beyond Cancer
Dr. Gang Zheng
Professor, Department of Medical Biophysics, University of Toronto
30 April 2024
12:00 – 1:00 pm ET | 9:00 – 10:00 am PT
Porphysomes are liposome-like nanoparticles self-assembled from a single porphyrin-lipid building block, which enables their inherent multifunction of photothermal, photoacoustic, photodynamic, fluorescence, PET, SPECT, MRI, and drug delivery.
Since the discovery of porphysomes, the Zheng Lab has demonstrated their high tumor selectivity and multimodal theranostic utilities in diverse tumor models and animal species. The Lab has completed GMP manufacturing, GLP safety studies and clinical trial protocols for its first-in-human use, aka ‘beyond lab’.
Dr. Zheng and colleagues have also developed a suite of next-generation porphysomes that greatly broadened their theranostic applications from light to sound to radiation. These new porphysomes will enable the pursuit of new directions ‘beyond light’ and ‘beyond cancer’.
Dr. Gang Zheng is a Senior Scientist and Associate Research Director of the Princess Margaret Cancer Center. He is also a Professor and Tier 1 Canada Research Chair in Cancer Nanomedicine at the University of Toronto. He obtained his BSc in 1988 from Hangzhou University and PhD in 1999 from SUNY Buffalo. Following a postdoctoral training in photodynamic therapy at the Roswell Park Cancer Institute, he joined the University of Pennsylvania in 2001 as an Assistant Professor of Radiology and moved to Canada in 2006. His lab is best known for introducing the activatable photosensitizers for photodynamic therapy and the porphysome nanotechnology for cancer theranostics. Dr. Zheng is a Fellow of the American Institute of Medical and Biological Engineering and an Associate Editor for Bioconjugate Chemistry.

Dr. Gang Zheng
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lectures
Next-Generation Lipid Nanoparticles for mRNA Delivery
Dr. Bowen Li
Assistant Professor, Leslie Dan Faculty of Pharmacy, University of Toronto
16 April 2024
12:00 – 1:00 pm ET | 9:00 – 10:00 am PT
The ability to transfect selective cell types within the targeted tissue in vivo is critical for potential therapeutic applications of mRNA. Although great advances have been made in mRNA vaccines, the ideal chemical and formulation composition of lipid nanoparticles (LNPs) for extra-hepatic delivery of nucleic acids are largely unknown. The traditional development of new lipids and formulations has been challenging, given the complexity of biological systems. A “high throughput” approach can be valuable because many variables can simultaneously be tested.
In this talk, Dr. Li will introduce a high-throughput combinatorial platform where thousands of chemically diverse libraries of lipid-like materials can be rapidly synthesized using multicomponent reactions and formulated into LNPs, which can then be screened for tissue- or cell-specific gene delivery. This platform technology increases the diversity of synthetic material structures and facilitates the identification of structure-function relationships.
Dr. Bowen Li is a tenure-track Assistant Professor in the Leslie Dan Faculty of Pharmacy at the University of Toronto and an Affiliate Scientist at Princess Margaret Cancer Centre. He holds a Canada Research Chair (Tier 2) in RNA Vaccines and Therapeutics and the GSK Chair in Pharmaceutics and Drug Delivery.
Dr. Li received his Ph.D. in Bioengineering from the University of Washington, Seattle, and then completed a Postdoc Fellowship under the guidance of Profs. Robert Langer and Daniel Anderson at MIT. His lab utilizes a range of interdisciplinary strategies, including combinatorial chemistry, high throughput platforms, and AI-driven design of experiments, to develop new generations of delivery systems for RNA medicines.
His work has led to over forty publications in top-tier journals, such as Nat. Biotechnology, Nat. Materials, Nat. Biomedical Engineering, Nat. Medicine, Sci. Adv., among others, as well as eight patents. His research has been recognized by the Marsha Morton Early Career Investigator Award from Cystic Fibrosis Canada, the Rising Star in Biological, Medicinal, and Pharmaceutical Chemistry from the American Chemical Society, the Gairdner Early Career Investigator Award, the J.P. Bickell Medical Research Award, the Connaught New Researcher Award, and the Baxter Young Investigator Award.

Dr. Bowen Li
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lectures
Is there a link between ice cream, baby shampoo and mRNA vaccines?
Dr. Nicolas Bertrand
Associate Professor, Faculty of Pharmacy, Université Laval
26 March 2024
1:00 – 2:00 pm ET | 10:00 – 11:00 am PT
Ice cream, baby shampoo and mRNA vaccines all contain poly(ethylene glycol) (PEG), a synthetic polymer also used in many other pharmaceutical products. In this presentation, Dr. Bertrand will discuss his findings from research on animals and humans as to how mammals can produce antibodies that recognize the polymer.
Dr. Nicolas Bertrand , BPharm, PhD, is a pharmacist by training and an associate professor at the Faculty of Pharmacy of Université Laval. His laboratory focuses on understanding the pharmacology of nanomedicine, specifically how nanomedicines interact with complex biological environments. His team has developed various tools to monitor the fate of liposomes and polymer nanoparticles in rodents. The laboratory is also very interested in understanding the intricate interactions between nanomaterials and the immune system.

Dr. Nicolas Bertrand
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lectures
Building Tools at the Interface between Synthetic Biology and Nanomedicine
Dr. Keith Pardee
Canada Research Chair in Synthetic Biology in Human Health & Associate Professor in the Leslie Dan Faculty of Pharmacy at the University of Toronto
3 November 2023
11:00 am – 12:00 pm PDT | 2 – 3:00 pm EDT
Our lab works in the area of synthetic biology, with an emphasis on human health. In this talk, I will discuss how we are working to develop engineered biological systems that interact with nanomaterials. This includes developing cell-free biomanufacturing technologies for the synthesis of proteins to functionalize nanoparticles, as well as the conjugation of biologics to develop new reporter systems for diagnostics.
Dr. Keith Pardee is the Canada Research Chair in Synthetic Biology in Human Health and is an Associate Professor at the Leslie Dan Faculty of Pharmacy at the University of Toronto (UofT). His lab works in the field of synthetic biology, and specifically is focused on pioneering in vitro devices to host cell-free synthetic gene networks for broad applications in human health. To do this, the lab uses freeze-dried enzymes of transcription and translation, and genetically encoded tools are embedded into porous materials, such as paper. Using this approach, they have created engineered platforms for low-cost diagnostics (e.g. Zika, COVID-19) and the portable production of protein-based therapeutics and lab reagents. Ongoing work is dedicated to the continued development of strategies to de-centralize access to tools for research and health care.

Dr. Keith Pardee
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
Pieter Cullis Invitational Lectures
Enablers of a new wave of genetic medicine
Dr. Kathryn Whitehead
Professor, Chemical Engineering and Biomedical Engineering, Carnegie Mellon University
4 October 2023
11 am – 12 pm PDT | 2 – 3 pm EDT
Messenger RNA (mRNA) therapeutics have taken center stage thanks to the successful deployment of the SARS-CoV2 mRNA vaccines in hundreds of millions of people worldwide. These vaccines were made possible by a herculean effort to overcome the most significant barriers that have hindered translational efforts. Arguably, the largest challenge has been that RNA molecules do not readily enter their cellular targets within the body. This is because they are large (104 – 106 g/mol) and negatively charged; they do not have favorable biodistribution properties nor an ability to cross the cell membrane of target cells.
In response to these issues, industrial and academic laboratories, including my own, have created lipid nanoparticles that spontaneously package RNA and deliver the RNA to key cellular targets in vivo. Here, I will describe biodegradable, ionizable lipid-like materials called ‘lipidoids’ that my lab has used to create RNA-loaded lipid nanoparticles that induce protein expression in a variety of tissues.
This talk will describe an especially potent lipid nanoparticle, its chemical characteristics that confer efficacy, and potential applications, including delivery to the pancreas. Together, these data advance our understanding of lipid nanoparticle chemistry and are expected to contribute to the successful formulation of next-generation mRNA therapies.
Dr. Kathryn Whitehead is a Professor in the Departments of Chemical Engineering and Biomedical Engineering (courtesy) at Carnegie Mellon University. Her lab develops drug delivery systems for RNA, proteins, and applications in maternal and infant health. She obtained bachelor and doctoral degrees in chemical engineering (Univ. of Delaware; Univ. of California, Santa Barbara) before an NIH Postdoctoral Fellowship at MIT.
Dr. Whitehead is the recipient of numerous awards, including the NIH Director’s New Innovator Award, the DARPA Director’s Fellowship, and the ASEE Curtis W. McGraw Research Award. She has also received the Controlled Release Society’s Young Investigator Award and served on its Board of Directors. Prof. Whitehead is an elected Fellow of the American Institute for Medical and Biological Engineering and the Controlled Release Society. In 2021, she gave a TED talk on the lipid nanoparticles (i.e., “fat balls”) used in the in the COVID-19 mRNA vaccines. Her publications have been cited over 10,000 times, and her patents have been licensed and sublicensed for reagent and therapeutic use.

The Pieter Cullis Invitational Lecture series features individuals working at the frontiers of nanomedicines research and innovation and showcases remarkable examples of scientific and commercial progress in the field.
NMIN
NMIN Lectures
Digital Microfluidics for Chemistry, Biology, and Medicine
This recording is available only to NMIN participants (formally affiliated researchers, trainees, partners, committee members, etc.) To access the full recording, please contact: info@nanomedicines.ca
Dr. Aaron Wheeler
Professor & Canada Research Chair, Bioanalytical Chemistry, University of Toronto
28 September 2023
11 am – 12 pm PDT | 2 – 3 pm EDT
Microfluidics is the study and application of fluid flow in microfabricated devices bearing length dimensions in the micrometer range (10-6 m, about the size of a human hair). This technology is revolutionizing the way we live, work, and interact, and in this presentation, I will describe microfluidic tools that my research group has developed to address problems in chemistry, biology, and medicine.
For chemistry, I will discuss our on-going work to initiate and evaluate chemical reactions and to screen for environmental perturbations within the tiny bores of super-conducting magnets in nuclear magnetic resonance spectrometers.
For biology, I will review our methods for isolating single cells to be able to profile their genomes, transcriptomes, and proteomes.
For medicine, I will summarize our work developing point of care diagnostic devices that can be operated in remote settings for serological surveillance of immune status. I will conclude with predictions about what the future may hold for this promising technology
Dr. Aaron Wheeler earned his Ph.D. in Chemistry at Stanford University, followed by an NIH postdoctoral fellowship at UCLA. In 2005, he joined the Department of Chemistry at the University of Toronto as an Assistant Professor, with cross-appointments in the Institute of Biomedical Engineering (IBME) and the Donnelley Centre for Cellular and Biomolecular Research (DCCBR). He was promoted to Associate and Full Professor in 2010 and 2013, respectively, and currently serves as the Tier 1 Canada Research Chair of Microfluidic Bioanalysis. Prof. Wheeler is the Editor-in-Chief of the flagship journal in the microfluidics community, Lab on a Chip, and in 2024 will co-host (with Prof. David Juncker, McGill University) the Micro Total Analysis (µTAS) conference, as it returns to Canada for the first time since 1998.

Dr. Aaron Wheeler
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lectures
Selective ablation of solid tumors using a p53-targeted FAST-LNP gene therapy
Dr. John Lewis
Professor, Department of Oncology, & Bird Dogs Chair in Translational Oncology, University of Alberta
18 July 2023
11:00 am PDT | 2:00 pm EDT
In this NMIN lecture, Dr. John Lewis will discuss a study in which he and his colleagues sought to develop an effective suicide gene therapy approach for solid tumors that specifically exploits their unique transcriptional activation state. The tumor suppressor p53 is frequently mutated or dysregulated in cancer, and as a result the upstream signaling pathways activating p53 transcription are strongly upregulated. RNA-seq analysis has demonstrated that p53 transcription is significantly upregulated in almost all forms of cancer. Additionally, HCT116 cells lacking functional p53 display a 6-fold increase in p53 promoter activity when compared to its wild type p53 parent cell line. To exploit this, Dr. Lewis and colleagues developed a Fusogenix FAST-LNP formulation to deliver a p53-driven inducible suicide gene, iCasp9, to solid tumors and destroy them upon activation with a small molecule dimerizer, Rapamycin.
Dr. John Lewis is a scientist, academic, and serial entrepreneur. His research interests include novel nanotechnology, nanoparticle drug delivery technologies, and imaging related to infectious diseases and chronic diseases such as aging and cancer.
As an academic, Dr. Lewis is a Professor and holds the Bird Dogs Chair in Translational Oncology at the University of Alberta and founded the Alberta Prostate Cancer Research Initiative. The chair is designated to drive the transformation of scientific discoveries into clinical applications that improve the diagnosis, treatment, survival, and quality of life of people with cancer. Dr. John Lewis’ academic laboratory studies metastatic cancers such as prostate cancer. Since no man dies of prostate cancer that stays in his prostate, the group uses real-time intravital imaging of the tumor microenvironment to understand cancer metastasis. The group has used this approach to identify key drivers of metastatic progression in prostate and other cancers, develop non-invasive tests to detect aggressive prostate cancer, and develop novel lipid nanoparticles to block the spread of prostate cancer.
As a serial entrepreneur, John Lewis founded and is the CEO of multiple Canadian biotechnology companies, including Entos Pharmaceuticals (Entos), a clinical-stage biotech company that developed an intracellular drug delivery platform called Fusogenix proteolipid vehicles (PLVs).

Dr. John Lewis
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lectures
Next-Generation RNA Vaccines & Therapies
Dr. Anna Blakney
Assistant Professor, Michael Smith Laboratories and School of Biomedical Engineering, The University of British Columbia
20 June 2023
8 – 9:00 am PDT | 11:00 am – 12:00 pm EDT
Dr. Anna Blakney is an Assistant Professor in the Michael Smith Laboratories and School of Biomedical Engineering at UBC. She received her Bachelor of Science in Chemical & Biological Engineering from the University of Colorado at Boulder, and her PhD in Bioengineering from the University of Washington. She completed a postdoctoral fellowship at Imperial College London on the development of molecular and biomaterial engineering strategies for delivery of self-amplifying RNA. Her lab uses bioengineering, molecular biology and immunology approaches to develop the next generation of RNA vaccines and therapies. She is also a passionate science communicator and runs a TikTok channel dedicated to educating the public about RNA biotechnology, which now has >250,000 followers and >18M views.

The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
Pieter Cullis Invitational Lecture
The quest for next-generation lipid nanoparticles for cell-specific delivery of mRNA
Dr. Gaurav Sahay
Associate Professor, Department of Pharmaceutical Sciences, Oregon State University
Friday, 24 March 2023
10 – 11:00 am PDT | 1 – 2:00 pm EDT
The field of nanomedicine is moving from an age of renaissance towards industrial revolution, in part due to the ability of lipid nanoparticles (LNPs) to package and deliver antigenic mRNA against SARS-CoV2, leading to the development of a powerful vaccine against COVID. The next horizon for our field remains tissue/cell-specific mRNA delivery that can lead to treatment of various diseases.
Our lab has worked extensively in LNP design, synthesis and structure and its impact on intracellular delivery of mRNA. I will talk about our lab’s efforts to understand the journey of an LNP-mRNA within the cellular interior, and how the fundamental insights we have attained led us to design nanoparticles that can deliver mRNA to different tissues for the treatment of cystic fibrosis and retinal degeneration, and as COVID-19 therapeutics.
We have further shown that novel peptide-guided LNPs can deliver mRNA selectively to the retinas of non-human primates and we plan to use mRNA-based genome editors for gene correction to treat blindness.
Finally, evolution of a cell-selective carrier for mRNA delivery will be discussed. Such platform technologies – with the capability to precisely deliver cargo to manipulate cells and correct diseases, with minimal off-target effects – will transform modern medicine.

Gaurav Sahay is an Associate Professor in the Department of Pharmaceutical Sciences and co-Director for the Center of Innovative Drug Delivery and Imaging (CIDDI), at the College of Pharmacy at Oregon State University. Dr. Sahay’s lab is developing novel nanotechnology-based platforms, including platforms using lipid-based nanoparticles, for the effective delivery of messenger RNA therapeutics to treat cystic fibrosis and retinal degeneration, and to protect against SARS-CoV2.
He has done pioneering work to dissect the intracellular transport essential for nucleic acid delivery to the cytosol and developed methods to overcome endosomal barriers. He has more than 60 peer-reviewed publications in top-tier journals including Science Advances, Nature, Nature Communications, Nature Biotechnology, Nature Nanotechnology, Journal of Controlled Release, Nano Letters etc. He is the winner of a 2013 American Association of Pharmaceutical Scientists (AAPS) Postdoctoral Fellow Award, the 2015 Controlled Release Society (CRS) T. Nagai Award, a 2016 American Association of Colleges of Pharmacy (AACP) New Investigator Award, a 2019 Oregon Health & Sciences University (OHSU) Distinguished Faculty Senate Award for Collaboration, 2020 Phi Kappa Phi OSU Emerging Scholar Award and 2020 CMBE Young Innovator Award.
He serves as the Principal Investigator on awards funded through the National Institutes of Health, Cystic Fibrosis Foundation, and biotech companies. He serves as a consultant and scientific advisory board member to several biotech and venture capital firms. He was the Chair of the 2018 NanoMedicine and Drug Delivery Symposium (NanoDDS, Portland, OR) and is standing section member for Innovative in NanoSystems and Nanotechnology. Dr. Sahay completed his postdoctoral research with Prof. Robert Langer and Prof. Daniel Anderson at the Koch Institute for Integrative Cancer Research at MIT and received his Ph.D. from the University of Nebraska Medical Center under the mentorship of Prof. Alexander Kabanov.
The Pieter Cullis Invitational Lecture series features individuals working at the frontiers of nanomedicines research and innovation and showcases remarkable examples of scientific and commercial progress in the field.
NMIN
NMIN Lecture
What about Copper and Immuno-Oncology?
Dr. Marcel Bally
Co-Leader of NMIN’s Research Theme 1: Targeted Drug Delivery & Leader of NMIN’s PharmaCore drug development platform facility; Head and Distinguished Professor, Experimental Therapeutics, BC Cancer
Thursday, 16 March 2023
12 – 1:00 pm PDT | 3 – 4:00 pm EDT
Dr. Bally’s BC Cancer Research team is pursuing a hypothesis that combinations of immunogenic Cell Death (ICD)—inducing drug candidates, enhanced by complexation with copper—will significantly improve the therapeutic benefits obtained following treatment with immune check point inhibitors (CPIs).
The use of immune CPIs is viewed as “game changing,” as many cancer patients with different types of cancer are now exhibiting long-term responses as judged by survival. Sadly, however, the benefits are seen in only a small subset of patients.
Dr. Bally’s lab is hoping that the long-term survival rate for patients that receive immune CPIs can double through the implementation of strategies that involve combinations with small molecular weight compounds that change the tumour micro-environment to one that is more immune supportive.
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.

Dr. Marcel Bally
Dr. Marcel Bally is Co-Leader of NMIN’s Research Theme 1: Targeted Drug Delivery and Leader of NMIN’s PharmaCore drug development platform facility; Head and Distinguished Scientist, Department of Experimental Therapeutics, BC Cancer; Member, Centre for Blood Research, The University of British Columbia (UBC); Professor, Pathology & Laboratory Medicine, UBC; and Adjunct Professor, Pharmaceutical Sciences, UBC. He has focused his career on development of novel drugs, drug combinations and drug delivery systems designed for use in patients with cancer.
Dr. Bally received his BSc (1977) and MSc (1979) degrees in biology from Texas A&M University. He obtained his PhD from the Department of Biochemistry at the University of British Columbia (1984).
He has recognized expertise in pharmacology/toxicology, drug formulations and preclinical cancer models, and is qualified to conduct preclinical safety studies under Good Laboratory Practices and has completed training in Good Manufacturing Practices.
His scientific works (scientific articles, abstracts, book chapters and patents) have been cited > 29,263 times. He has trained >70 highly qualified personnel, many of whom now hold significant positions in industry, academia and medical practice.
He has co-founded multiple companies including: Lipex (acquired by Northern Lipids); Inex (now Arbutus); Northern Lipids (renamed Transferra and purchased by Evonik in 2016); Celator (purchased by Jazz in 2016); and Cuprous Pharmaceuticals.
Dr. Bally was one of the co-founders of Canada’s NCE Centre for Drug Research and Development (joined with NEOMED to form adMare BioInnovations in 2019), as well as the Nanomedicine Innovation Network (NMIN). His research has contributed to the success of three marketed drugs: Myocet (for metastatic breast cancer); Marqibo (for relapsed ALL); and Vyxeos (for high risk AML).
NMIN
NMIN Lectures
Single-Particle Imaging to Quantitate Biophysical Properties of mRNA LNPs & Engineer Improved Vaccines & Therapies
Dr. Sabrina Leslie
Associate Professor, Michael Smith Labs and Physics Department, University of British Columbia
24 January 2023
11:00 am – 12:00 pm PT | 2:00 – 3:00 pm ET
Dr. Leslie will discuss a quantitative single-particle imaging platform that enables simultaneous measurements of the size, mRNA-payload, and dynamic properties of vaccines in cell-like conditions.
By directly imaging the trajectories of many single molecules simultaneously and in a dynamic manner, her Convex Lens-induced Confinement (CLiC) microscopy allows for the investigation of the design rules and mechanisms that govern how oligos (or proteins or drug molecules) interact with target sites on nucleic acids (Scott et. al, Nucl. Acid Research. 2018, 2019) and how molecular cargo is released inside cells from lipid nanoparticles (Kamanzi et al. ACS Nano 2021).
Dr. Leslie’s team investigates the dependence of mRNA-lipid-nanoparticle structure and fusion dynamics on formulation, using commercially available formulations as a starting point. These measurements are made on confined, freely diffusing particles and during reagent-exchange, such as in response to solution pH, to emulate intracellular dynamics in a controlled setting.
Over the long term and in collaboration with health scientists, Dr. Leslie and team propose to correlate multi-scale data sets—including single-particle measurements made in vitro as well as in cells and tissues—with clinical results, to create a throughline of understanding of vaccine effectiveness from the microscopic to the clinical scale, to enable and optimize their rational design and engineering.

Dr. Sabrina Leslie
Dr. Sabrina Leslie is working at the interface of physics and biology with a particular interest in quantifying the dynamics of individual molecules. Dr. Leslie’s research provides insights into the biophysical properties of molecules and cells, including therapeutics with the ultimate goal of propelling quantitative health sciences. Her team is developing single-molecule and single-cell imaging and analysis tools, as well as mathematical models of molecular interactions, to help guide the rational design of new therapeutic molecules and delivery vehicles.
Dr. Leslie studied honours physics and mathematics at the University of British Columbia 20 years ago before moving to UC Berkeley where she obtained her PhD in optical physics in 2008, followed by her Mary Fieser post-doctoral fellowship in biophysics at Harvard University 2009-2011. There she invented a tether-free, high-throughput single-molecule imaging technology called Convex Lens-induced Confinement (CLiC), which established her as a pioneer in single-molecule investigations.
In 2012, she became an Assistant Professor at McGill University and founded her research group in the Physics Department. There she developed CLiC into a platform technology and used it for new single-molecule studies of nucleic acids, proteins, polymers, nanomaterials, biologics and cells. In 2019 she was honored with the Young Investigator Award from the Biophysical Society of Canada, and in 2020 she was elected to the Royal Society of Canada (RSC) College of New Artists Scholars and Scientists.
In 2021, she and her team re-located to the Michael Smith Labs at UBC where her Associate Professor appointment is joint with the Department of Physics and Astronomy and affiliated with the School of Biomedical Engineering, Genome Science and Applied Technology and Bioinformatics programs.
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lectures
Lipid nanoparticle-mediated delivery of CRISPR components for neuronal genome editing
Dr. Blair Leavitt
Professor, Department of Medical Genetics & the Department of Medicine, Division of Neurology (Associate), The University of British Columbia
Sarah Thomson
PhD Candidate, The University of British Columbia
Tuesday, 29 November 2022
11:00 am – 12:00 pm PT | 2:00 – 3:00 pm ET
Lipid nanoparticle (LNP)-enabled gene therapy is a promising approach for the treatment of genetic neurological disease. To optimize LNP-enabled nucleic acid delivery for this purpose, we developed an iterative screening strategy using neurons ex vivo to identify unique LNP formulation parameters for siRNA and mRNA delivery. We show LNPs efficiently deliver both cargoes to neurons ex vivo, that formulation potency and toxicity vary with lipid composition and dose, and explore the utility of LNP-mediated CRISPR/Cas9 genome editing in neurons.
Dr. Blair Leavitt, MDCM FRCP(C) is a Huntington’s disease (HD) neurologist. He currently holds positions as a Senior Scientist at the Centre for Molecular Medicine and Therapeutics, and as a Professor in the Department of Medical Genetics at the University of British Columbia.
Dr. Leavitt is a long-standing member of NMIN, a former Co-Chair of the Huntington Study Group, an established HD clinical trial investigator, and the Director of Research at the UBC Centre for HD in Vancouver. He has an ongoing clinical research program in neurogenetics with a focus on HD. A laboratory scientist as well as a practicing neurologist, his laboratory is dedicated to developing and testing new treatments for genetic diseases of the brain and spinal cord. Using genetically-modified mouse models of human disease as his primary tool, Dr. Leavitt’s research focuses on developing new therapies for devastating neurodegenerative diseases such as HD, FTD and ALS. His research also includes research programs searching for clinical biomarkers in HD and identifying new genetic causes of ataxia, epilepsy, neurodevelopmental disorders, and autism.
He is the co-founder and CEO of Incisive Genetics Inc. a biotech company developing lipid nanoparticle delivery systems for gene editing applications. Dr. Leavitt is a founding Co-Editor-in-Chief of the Journal of Huntington’s Disease.
Sarah Thomson is a PhD candidate in Dr. Blair Leavitt’s research group at the University of British Columbia. Her research is focused on transcriptional regulation in Huntington’s disease, and the therapeutic modulation of gene expression using nucleic acid effectors in the brain.

Dr. Blair Leavitt

Sarah Thomson
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
Pieter Cullis Invitational Lecture
DNA Nanostructures: From Design to Biological Function
Dr. Hanadi Sleiman
Professor & Canada Research Chair in DNA Nanoscience, Dept of Chemistry, McGill University; Associate Editor, Journal of the American Chemical Society
Tuesday, 20 September 2022
11:30 am – 12:45 pm PT | 2:30 – 3:45 pm ET
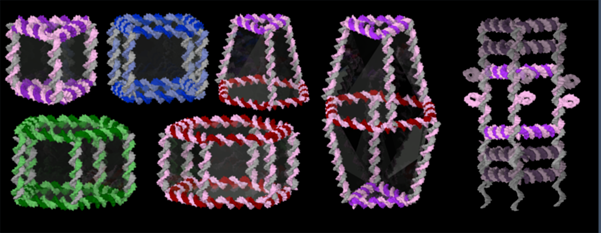
We recognize DNA as the fundamental building block of life, the blueprint that defines who we are. But the very properties that make DNA such a reliable molecule for information storage also make it a remarkable building material.
Over the past few years, Dr. Sleiman’s research group has taken DNA out of its biological context and has used it to build nanostructures for applications in biology and materials science. Starting from a minimum number of DNA components, her group creates 3D-DNA host structures, such as cages, nanotubes and spherical nucleic acids that hold great promise for achieving targeted drug delivery. These structures can be precisely controlled in size, shape, and presentation of molecules on their surface; they can load drug cargo and deliver it on demand, in response to disease-specific biological triggers. Her team finds that these DNA structures resist nuclease degradation, silence gene expression, and have a favorable in vivo distribution profile.
In this lecture, Dr. Sleiman will describe the application of these DNA structures as drug delivery vehicles to cancer cells. She will also discuss the ability of small molecules to completely reprogram the assembly of DNA, allowing for new motifs of DNA assembly that go beyond the established technique of Watson-Crick-Franklin base-pairing.
References:
- N. Seeman, H. Sleiman, DNA Nanotechnology, Nat. Rev. Mat., 2017, 17068.
- F. Rizzuto, C. Platnich, X. Luo, M. Dore, C. Lachance-Brais, G. Cosa, H. Sleiman, A dissipative pathway for the structural evolution of DNA fibers, Nat. Chem., 2021, 13, 843–849.
- M. Dore, T. Trinh, D. de Rochambeau, P. Xu, J. Li, H. F. Sleiman, Chem, 2021, 7, 2395-2414.
- K. E. Bujold, J. C. C. Hsu and H. F. Sleiman; Optimized DNA “Nanosuitcases” for Encapsulation and Conditional Release of siRNA, J. Am. Chem. Soc. 2016, 138, 14030–14038.

Dr. Hanadi Sleiman, PhD, is a Professor of Chemistry and Canada Research Chair in DNA Nanoscience at McGill University. She received her Ph.D. from Stanford University, and was a CNRS postdoctoral fellow in Prof. Jean-Marie Lehn’s laboratory at the Université Louis Pasteur. Her research group focuses on using the molecule DNA as a template to assemble nanostructured materials for drug delivery and diagnostics.
Dr. Sleiman is Fellow of the Royal Society of Canada (2016), Associate Editor of J. Am. Chem. Soc., and Editorial Advisory Board member of J. Am. Chem. Soc., Chem., J. Org. Chem., and ChemBioChem.Among her recent research recognitions are the NSERC Polanyi Award (2021), Research Corporation Cottrell STAR award (2021), Killam Research Fellowship (2018), CSC R. U. Lemieux Award (2018), Netherlands Scholar Award in Supramolecular Chemistry (2018), Izatt-Christensen Award in Supramolecular Chemistry (2016), Swiss Chemical Society Lectureship (2012), CIC E. Gordon Young Award (2011), CSC Strem Award (2009). She received the McGill Principal’s Prize and the Leo Yaffe Award for Excellence in Teaching.
The Pieter Cullis Invitational Lecture series features individuals working at the frontiers of nanomedicines research and innovation and showcases remarkable examples of scientific and commercial progress in the field.
NMIN
NMIN Lectures
Development & utilization of in vivo systems to optimize lipid nanoparticles for therapeutic genome editing
Dr. Colin Ross
Associate Professor in the Faculty of Pharmaceutical Sciences at the University of British Columbia
29 July 2022
12-1 pm Pacific | 3-4 pm Eastern
Genetic diseases are a leading cause of death and disability in Canada with immense economic and societal burdens. Gene therapy has emerged as a means to effectively treat genetic diseases; however, current gene therapies are limited by their high manufacturing costs, the inability to re-dose, and the safety concerns of some viral vectors.
CRISPR genome editing is a new therapeutic approach that aims to directly repair the underlying disease-causing mutations. Conventional CRISPR methods are limited as in vivo therapeutics because they introduce DNA breaks and cause frequent off-target edits. Newer base editors and prime editors overcome the limitations traditional CRISPR genome editing methods because they do not introduce DNA breaks.
However, the delivery of genome editors to affected tissues remains a challenge. Viral vectors, such as AAV, are unsuitable for genome editing because their long expression (years) increases the probability of unintended edits. In contrast, the transient expression (hours-days) of RNA encoding genome editors via nanoparticles is well suited for genome editing, and unlike viral vectors, nanoparticles can be readministered. However, nanoparticle delivery of complex genome editing cargos (large mRNA + small gRNA) remains a challenge, especially to extra-hepatic target tissues such as muscle. To address this, we are developing new ways to safely deliver these new editors using lipid nanoparticles.
To efficiently measure the in vivo effectiveness of genome editor delivery via LNPs, we have developed transgenic mice that carry mutations in reporter genes. Precise gene repair of these mutations produce a functional enzyme that emits light (luminescence) that sensitive imagers can detect to precisely measure the location and extent of gene editing in living animals. We have made progress in our goal towards efficient and safe in vivo genome editing that we will share.
Dr. Colin Ross seeks to incorporate genomics into guiding, optimising, and developing novel therapeutics to improve the safety and effectiveness of medications. His research is also exploring the use of genome sequencing to help diagnose and manage the treatment of rare genetic diseases.

Dr. Colin Ross
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
Pieter Cullis Invitational Lecture
Scientific Integrity Series
What has gone wrong with publication practices?
Prof. Dr. Jean-Christophe Leroux
Professor in Drug Formulation & Delivery and Head, the Institute of Pharmaceutical Sciences, the Swiss Federal Institute of Technology (ETH Zürich)
Tuesday 31 May 2022
9-10:15 am PST | 12-1:15 pm EST
As in several other scientific disciplines, pharmaceutical sciences, and in particularly nanomedicine is experiencing a reproducibility crisis [1]. This crisis has multiples roots, and can be in part explained by the use of ever more complex experimental protocols and specialized systems [2], inaccurate or incomplete description of methods employed and lack of rigor in the design of experimental plan. However, another aspect that should not be ignored, and that possibly contributes to exacerbating this problem is the drift which is currently occurring in the publication practices, where for some groups, sharing scientific findings ceases to be the primary reason for publishing their research findings. Using examples taken mainly from the field of drug delivery, this presentation aims at analyzing the subtle causes that may contribute to the lack of reproducibility of the scientific literature and its impact on the society. It will also provide some possible solutions to disseminate new knowledge in a more reliable fashion.
References:
[1]. Leroux JC, Angew. Chem. Int. Ed. 2017, 56, 15170-15171.
[2]. Leroux JC, J Control. Release 2018, 278, 140-141.
Prof. Dr. Jean-Christophe Leroux is a full professor of Drug Formulation and Delivery and head of the Institute of Pharmaceutical Sciences at the ETH Zurich, Switzerland. He has made important fundamental and applied contributions to the fields of biomaterials and drug delivery, and has been involved in the development of innovative bio-detoxification systems for the treatment of metabolite disorders. He is a fellow of the AAPS, EURASC and the CRS, and the co-founder of the start-up pharmaceutical companies Versantis AG and Inositec AG.

Prof. Dr. Jean-Christophe Leroux
ETH Zurich, Switzerland
The role of validation & retraction in upholding scientific integrity
a webinar with
Dr. Ivan Oransky of Retraction Watch
The Pieter Cullis Invitational Lecture series features individuals working at the frontiers of nanomedicines research and innovation and showcases remarkable examples of scientific and commercial progress in the field.
NMIN
NMIN Lectures
Cancer nanomedicine: Using gold nanoparticles to overcome radiotherapy challenges
Dr. Devika Chithrani
Associate Professor, University of Victoria
Friday, 20 May 2022
11:00 am – 12:00 pm PDT | 2:00 – 3:00 pm EDT
Globally, Cancer is the second leading cause of death. In 2018, there were 18.1 million new cases worldwide and 9.5 million cancer-related deaths. By 2040, the number of new cancer cases per year is expected to rise to 29.5 million and the number of cancer-related deaths to 16.4 million. Approximately 50 percent of all cancer patients can benefit from radiotherapy (RT) in the management of their disease. Of these, approximately half present early enough to pursue curative treatment approaches.
The major limitation to reaching a curative RT dose in high-risk (locally advanced) non-metastatic tumors is the high sensitivity to radiation and subsequent damage to the surrounding normal tissues. In an effort towards reducing side effects while increasing the damage to the tumour, targeting of high atomic number materials such as gold nanoparticles (GNPs) as radiosensitizers to the tumour tissue has shown promising results.
Moving forward, understanding of the complex biological system present in and around the tumour is essential for optimizing the use of the radiosensitizing GNPs, as outlined by a consortium of labs, including my own. In this talk, I will discuss the importance of using GNP-based novel strategies to overcome current challenges imposed by the tumour microenvironment.
Dr. Devika Chithrani is an associate professor in the Department of Physics and Astronomy at the University of Victoria, Canada. She was awarded the faculty gold medal and the gold medal for physics when she received her bachelor’s degree (first class honors). To continue her graduate studies, she was awarded a prestigious NSERC Graduate scholarship in materials science and engineering at University of Toronto. Following successful completion of her doctoral work, she was awarded one of the most prestigious awards in Canada, the NSERC PDF, to continue her post-graduate research at the University of Toronto.
Dr. Chithrani now leverages nanotechnology to create innovations that advance the care of cancer patients. She is using gold nanoparticles as a radiation dose enhancer in cancer therapy. This work was featured on the cover of the journal Radiation Research and received the Michael S. Patterson publication award. She has developed three-dimensional tumor models to optimize bio-nano interface in cancer therapy. This work is featured on the cover of the journal Nano-Micro Letters.
Dr. Chithrani is considered one of the leaders in the field of nanotechnology and her publications have received over 10,000 citations over the past 10 years. Her passion is to develop smart nanomaterials to improve exiting cancer therapeutics. She believes that many side effects due to chemotherapy can be reduced by controlled delivery of anticancer drugs using smart nanomaterials.

Dr. Devika Chithrani
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
Pieter Cullis Invitational Lecture
Liposomes – Six Decades On: The Old Kid On The Cancer Nanomedicine Block
Dr. Francis Szoka
Professor in the Department of Bioengineering and Therapeutic Sciences of the University of California San Francisco
Wednesday 20 April 2022
10-11:00 am PST | 1-2:00 pm EST
I come before you to praise liposomes… not to bury them.
As we approach the sixtieth anniversary of the first description of a liposome by Alec Bangham, I will briefly reflect upon the reasons for the successes of liposome encapsulated drugs in cancer drug therapy. More relevant to the future, as many others have pointed out, we need to comprehend why liposomes haven’t been more successful clinically and what this implies for the broader research area of nanomedicines.
This presentation will focus on relevant results from our group in three areas: drug encapsulation and release, long circulation, and tissue and tumor penetration. I think there remain challenging, unresolved research opportunities for curious scientists to understand and exploit for improved cancer nanomedicine delivery.
Bangham, A. D.; Horne, R. W. (1964). “Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents As Observed in the Electron Microscope“. Journal of Molecular Biology. 8 (5): 660–668.
Dr. Francis Szoka focuses on improving gene, recombinant protein and drug delivery using biophysical, molecular biology and chemical approaches. He and his team have applied biophysical approaches to create methods and tools that are now widely used to manufacture liposomes, polyplexes and lipoplexes. They have extensively investigated the mechanisms of non-viral gene delivery. Their recent efforts in the treatment of cancer involves the design and synthesis of prodrugs and the generation of amorphous nanosized drug particles to improve the bioavailability of sparingly soluble anticancer drugs.

Dr. Francis Szoka
The Pieter Cullis Invitational Lecture series features individuals working at the frontiers of nanomedicines research and innovation and showcases remarkable examples of scientific and commercial progress in the field.
NMIN
NMIN Lectures
Porphysomes and beyond – the path to clinical translation
Dr. Gang Zheng
Professor, Department of Medical Biophysics, University of Toronto
Wednesday, 23 March 2022
10:00 – 11:00 am PDT | 1:00 – 2:00 pm EDT
Dr. Gang Zheng is Professor, Department of Medical Biophysics, University of Toronto, cross-appointed to Institute of Biomaterials and Biomedical Engineering & the Leslie Dan Faculty of Pharmacy & the Institute of Medical Science. He is also Associate Research Director of Princess Margaret Cancer Centre.
His research is currently focused on developing clinically translatable technology platforms to combat cancer. His lab discovered porphysome nanotechnology (Nature Materials 2011), named one of the “top 10 cancer breakthroughs of 2011” by the Canadian Cancer Society. His lab also discovered that on exposure to low-frequency ultrasound, porphyrin microbubbles form nanoparticles that possess the same optical and therapeutic properties as the original microbubble, and can be used simultaneously for imaging and drug delivery (Nature Nano 2015). Dr. Zheng is an Associate Editor for Bioconjugate Chemistry and a Fellow of the American Institute for Medical and Biological Engineering. In 2019, he was awarded a Tier 1 Canada Research Chair in Cancer Nanomedicine.

Dr. Gang Zheng
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lectures
Lipidic nanoparticle formulations of a triple adjuvant for intranasal mucosal vaccines
Dr. Ellen K. Wasan
Associate Professor, College of Pharmacy and Nutrition, University of Saskatchewan
Friday, 11 February 2022
11:00 am – 12:00 pm PST | 2:00 – 3:00 pm EST
Dr. Ellen K. Wasan, B.S.Pharm., R.Ph., Ph.D., is an Associate Professor in the College of Pharmacy and Nutrition at the University of Saskatchewan. She is part of the Drug Design and Delivery research cluster and teaches in the Doctor of Pharmacy program. Dr. Wasan received her PhD from the University of British Columbia in 1999 and worked as a research scientist at the BC Cancer Research Centre, followed by a faculty position in the Dept. of Basic Health Sciences at the British Columbia Institute of Technology. Her laboratory research is funded by NMIN, the Government of Saskatchewan Agricultural Research Branch, the Saskatchewan Cattlemen’s Association, and the Canadian Institutes of Health Research, in collaboration with the Vaccine and Infectious Disease Organization (VIDO-InterVac). She is currently Secretary of the Board for the Canada Chapter of the Controlled Release Society, a member of the Canadian Society for Pharmaceutical Sciences and is a practicing community pharmacist.
Dr. Wasan’s research in pharmaceutics has focused on formulation of poorly water-soluble drugs, lipid-based drug and nucleic acid delivery and advanced drug delivery systems such as nanoparticles for applications in cancer and infectious disease. Currently her research group is working on nanoparticle vaccines, oral sustained release polymeric nanoparticles and novel therapies for rheumatic disorders. Her laboratory research is funded by NMIN, the Government of Saskatchewan Agricultural Research Branch, the Saskatchewan Cattlemen’s Association, and the Canadian Institutes of Health Research, in collaboration with the Vaccine and Infectious Disease Organization (VIDO-InterVac).
Dr. Ellen K. Wasan
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
Pieter Cullis Invitational Lecture Series
Designing nanomaterials for therapeutics and biosensing
Dr. Molly Stevens
Professor of Biomedical Materials and Regenerative Medicine in the Department of Materials and the Department of Bioengineering, and Research Director for Biomedical Material Sciences at the Institute of Biomedical Engineering, Imperial College London
Tuesday 28 September 2021
10-11:00 am PDT | 1-2:00 pm EDT
This talk provided an overview of recent work by Dr. Molly Stevens and the Stevens Group at Imperial College London using designer bio-nanomaterials for biosensing and controlled delivery.
Dr. Stevens and her Group are exploiting the sensing capabilities of nanoparticles to engineer detection assays for infectious diseases such as HIV, Ebola, tuberculosis and COVID-19, and they are integrating these capabilities with smartphone technology to enable patient self-monitoring, geographical tagging and epidemic surveillance. They are also engineering complex 3D architectures and cell interfacing nanoneedles for multiplexed intracellular biosensing and modulation of biological processes. Their in-depth research on liposomal platforms using neutron scattering is informing the choice of lipid composition and formulation method for biological applications. For example, they have tailored lipid-based nanocarriers for ultrasound-triggered hydrogelation to enable in vivo remote remote-triggered drug delivery.
In this lecture, Dr. Stevens discussed how the versatile technologies she is developing with the Stevens Group can be applied to transformative biosensing, regenerative medicine and new therapeutic approaches.

Imperial College London
Molly Stevens is Professor of Biomedical Materials and Regenerative Medicine in the Department of Materials and the Department of Bioengineering, and the Research Director for Biomedical Material Sciences at the Institute of Biomedical Engineering, at Imperial College London.
The Stevens Group is a large, multidisciplinary research group of students, postdocs and research fellows who use innovative bioengineering approaches to pursue their vision of solving key problems in regenerative medicine and biosensing. The Group’s research spans drug delivery, bioactive materials, tissue engineering, biosensing, materials characterisation, soft robotics and the interface between living and non-living matter, and is underpinned by collaborations with data scientists and molecular dynamics experts.
Dr. Stevens is Fellow of eight UK Societies including the Royal Society and the Royal Academy of Engineering. In 2019, she was elected Foreign Member of the National Academy of Engineering (USA). She holds numerous leadership positions, including: Director of the UK Regenerative Medicine Platform “Smart Materials” Hub; Deputy Director of the EPSRC-funded Interdisciplinary Research Centre in Early-Warning Sensing Systems for Infectious Diseases; Associate Director of the British Heart Foundation Centre of Research Excellence; previous member of the World Economic Forum Global Future Council for Advanced Materials; Scientist Trustee of the National Gallery (London, UK); and Associate Editor of ACS Nano and previous reviewing editor at Science.
Dr. Stevens has received over 30 prestigious awards, including the Acta Biomaterialia Silver Medal (2020), Surfaces and Interfaces Award (Royal Society of Chemistry 2019), Rosalind Franklin Medal and Prize (Institute of Physics, 2018), the Harrison Medal (Royal Pharmaceutical Society, 2017) and the Imperial College President’s Award and Medal for Outstanding Research Team (2016).
The Pieter Cullis Invitational Lecture series features individuals working at the frontiers of nanomedicines research and innovation and showcases remarkable examples of scientific and commercial progress in the field.
NMIN
NMIN Lectures
Lipid and RNA Nanomedicines to Control Bleeding and Thrombosis
Dr. Christian Kastrup
Associate Professor in the Michael Smith Laboratories and Department of Biochemistry & Molecular Biology at the University of British Columbia; member of the Centre for Blood Research and the School of Biomedical Engineering
Tuesday 21 September 2021
10-11:00 am PDT | 1-2:00 pm EDT
Blood coagulation is necessary to stop bleeds, but an imbalance of the enzymes that form, inhibit and degrade blood clots can lead to bleeding disorders, failure to stop severe hemorrhage after injury, or formation of thromboses. Gene therapy, using RNA and lipid nanoparticles, can be used to modulate the concentration of these enzymes and correct any imbalance. This talk highlights the applicability of RNA and lipid nanoparticles to control the expression of endogenous and exogenous proteins in the liver and in platelets, toward the goal of creating useful therapies for trauma, rare bleeding disorders, and thrombosis.

Dr. Christian Kastrup
Dr. Christian Kastrup did his postdoctoral fellowship in at MIT, specializing in engineering biomaterials for cardiovascular drug delivery. He received his PhD at the University of Chicago, specializing in chemical biology, microfluidics, and blood coagulation. His lab at UBC utilizes biochemical engineering to solve problems related to hemostasis and hemorrhage. They investigate, utilize, and mimic the biochemistry and biophysical dynamics of blood coagulation to create innovative materials that perform new functions inside of blood vessels, and work to develop treatments for severe hemorrhage.
Dr. Kastrup has received many accolades, the most recent being the Sir Major Banting Award from the True Patriot Love Foundation. He is the Chief Scientific Officer of CoMotion Drug Delivery Systems, Inc., which is working to develop hemostatic agents for severe combat and surgical hemorrhage.
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lecture Series
Nano-delivery of Novel Inhibitors of DNA Repair for Enhanced Cancer Therapy:
Making a case for the use of nano-medicine in the drug development process
Dr. Afsaneh Lavasanifar
Professor of Pharmaceutical Sciences, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta; Scientific Chief Officer and Vice President, Meros Polymers Inc.
Nanomedicine is commonly developed to either correct the undesired effects of therapeutic agents already in clinic or enhance the delivery of challenging molecules, such as proteins and genes, to their cellular targets. In this project, our aim is to harness the benefits of nanomedicine in the drug development process towards making “new drugs” for cancer therapy.
In this context, polymer as well as lipid-based nano-delivery systems for the solubilization and delivery of new small molecule inhibitors of a DNA repair enzyme, known as polynucleotide kinase 3′-phosphatase (PNKP), have been developed. Our preclinical assessments, so far, have provided evidence for the success of a polymer-based nano-formulation of a hit PNKP inhibitor, A83B4C63, as novel synthetically lethal nano-therapeutics in the treatment of colorectal cancers (CRC) deficient in Phosphatase and TENsin homolog deleted on chromosome 10 (PTEN). Successful use of this formulation as a radiosensitizer has also been shown in wildtype PTEN positive CRC models. Research on the evaluation of the biological activity of a liposomal formulation of another “hit” PNKP inhibitor, A83B47C63, and its head-to-head comparison with polymeric nanoparticles of A83B4C63 is ongoing.

Dr. Afsaneh Lavasanifar is Professor in the Pharmaceutical Sciences division of the Faculty of Pharmacy and Pharmaceutical Sciences at the University of Alberta. She is also the Scientific Chief Officer and Vice President of Meros Polymers Inc., a spinoff company established on basis of technology developed her lab.
Her research is focused on the design and development of polymer-based delivery systems that can increase solubility, modify the pharmacokinetic pattern, reduce toxicity and increase the efficacy of different therapeutic agents. The ongoing research projects in her laboratory include development of novel polymeric nano-carriers and stimulus responsive gels for application in cancer chemo and immunotherapy or deliver of anti-inflammatory agents. She is an inventor in 5 patent/patent applications on novel polymer-based formulations for drug and siRNA delivery.
Dr. Lavasanifar is the Associate Editor of Molecular Pharmaceutics and a member of the Editorial Board of Materials Sciences and Applications and Iranian Polymer Journal.
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
NMIN Lecture Series
The nanoparticle biological identity & protein corona: Challenges, opportunities, & future research directions
Dr. Warren Chan
Professor, Canada Research Chair in Nanobioengineering & Director, Institute of Biomedical Engineering (BME), University of Toronto
Monday 12 April 2021
1:00 – 2:00 pm PDT | 4:00 – 5:00 pm EDT
Dr. Chan’s Integrated Nanotechnology & Biomedical Sciences Laboratory is interested in studying and understanding the proteomic and genomic changes associated with abnormal cells (e.g., cancer cells or virally-infected cells) and tissues. His lab aims to elucidate the cell’s molecular dynamics by using recent developments in nanotechnology (e.g., inorganic nanostructures), microtechnology (e.g., micro-electromechanical systems and capillary flow systems), and molecular engineering (e.g., phage-display) as well as engineering new instrumentation and techniques to address biological questions. Dr. Chan and colleagues seek a fundamental understanding of molecular processes with technology developments toward the designing of novel diagnostic schemes and therapeutic strategies.

Dr. Warren Chan
Warren Chan, PhD, is Director of the Institute of Biomaterials and Biomedical Engineering; Distinguished Professor of Nanobioengineering; and Canada Research Chair in Nanobioengineering – all at the University of Toronto. He is also an NMIN Principal Investigator in Theme 3: Diagnostics.
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.
NMIN
Pieter Cullis Invitational Lecture Series
Biomaterials for the Delivery
of Nucleic Acids, Genome Editing Tools and Cells
Dr. Daniel G. Anderson
Professor of Chemical Engineering, Institute for Medical Engineering and Science, Koch Institute for Integrative Cancer Research, and the Harvard-MIT Division of Health Science and Technology at MIT
Daniel G. Anderson is a Professor at the Massachusettes Institute of Technology (MIT) in the Department of Chemical Engineering, Institute for Medical Engineering and Science, Koch Institute for Integrative Cancer Research, and the Harvard-MIT Division of Health Science and Technology.
The research done in Prof. Anderson’s laboratory is focused on developing new materials for medicine. He has pioneered the development of smart biomaterials, and his work has led to advances in a range of areas, including medical devices, cell therapy, drug delivery, gene therapy and material science.
Prof. Anderson received a B.A. in mathematics and biology from the University of California at Santa Cruz and a Ph.D. in molecular genetics from the University of California at Davis. His work has resulted in the publication of over 400 papers, patentsand patent applications. These advances have led products that have been commercialized or are in clinical development, as well as to the foundation of companies in the pharmaceutical, biotechnology, and consumer products space.
Dr. Anderson is a founder of Living Proof, Olivo Labs, Crispr Therapeutics (CRSP), Sigilon Therapeutics, Verseau Therapeutics, Orna, and VasoRx.

Dr. Daniel G. Anderson
The Pieter Cullis Invitational Lecture series features individuals working at the frontiers of nanomedicines research and innovation and showcases remarkable examples of scientific and commercial progress in the field.
NMIN
NMIN Lecture Series
If I Wasn’t a Scientific Entrepreneur
We Wouldn’t Have Enabled the Pfizer/BioNTech COVID-19 Vaccine
Dr. Pieter Cullis
Scientific Director & CEO, The NanoMedicines Innovation Network
Professor, Department of Biochemistry & Molecular Biology, The University of British Columbia
Tuesday 23 February 2021
9 – 10:00 am PST | 12 – 1:00 pm EST
During the inaugural NMIN Lecture, Dr. Pieter Cullis recounted the history of the research that led to the lipid nanoparticle (LNP) delivery system enabling the Pfizer/BioNTech COVID-19 vaccine, and discussed why being a scientific entrepreneur was vital to this success.
He also spoke about future opportunities and challenges in nanomedicines, and suggested ways Canada might remain at the forefront of this field.

Dr. Pieter Cullis
The NMIN Lecture Series focuses on the activities and successes of NMIN investigators and/or Network research. These lectures provide opportunities for Network participants and stakeholders to stay up to date with Network research, and promote collaboration between Network labs. Attendance is open to any interested parties.